Life-Threatening Non-ACS Chest Pain
Life-Threatening Non-ACS Chest Pain
Not all serious chest pain will be due to ACS. Responders must be aware of other causes of chest pain that will need emergent treatment. While many are easily managed chronically, distinguishing those that are life-threatening will be critical in saving patients’ lives.
Aortic Dissection
While this condition is much less common than ACS, it is very dangerous to the patients. Approximately 30 patients in one million will have an aortic dissection compared to 4400 patients in one million for acute MIs. On the other hand, about 25% of aortic dissection patients will die within 24 hours, and the rest will die within 2 weeks if not treated.
The most common symptom of the disease is severe chest pain. The risk for the disease includes bicuspid aortic valves, aortitis, Marfan’s and other connective tissue disorders, male gender, trauma to the chest, hypertension, older age (60s and 70s), as well as cocaine use. It can be an adverse result of cardiac catheterization.
The classic patient is a hypertensive male in the eighth decade who complains of severe and sudden chest pain that reaches its apex at the onset. The patient may describe “ripping” pain. Note that this can be a very similar presentation to ACS. Chest pain can move over time and can give a clue as to the location of the dissection. Chest pain that is primarily anterior may suggest ascending or transverse location, while associated posterior and abdominal pain may indicate a descending dissection. As always, classical presentation is not universal; only half will have typical “ripping” pain, while almost one third may have an aortic regurgitation murmur, and almost 20% will have an abnormal pulse.
ECG changes
There may be a range of findings on the EKG. About ¼ will have signs of LVH due to hypertensive history. There may be ST or T wave changes due to the repolarization abnormalities of LVH as well. Note that ST elevation can even occur due to the obstruction of coronary arteries from the dilated aortic root. Consequently, EKGs are not typically useful for diagnosing aortic dissection or differentiating it from ACS.
Pulmonary Emboli
Pulmonary emboli (PE) is due to thrombotic events that usually begin in the legs. These thrombi can break off pieces that then travel to the right side of the heart to the pulmonary arteries. The symptoms will depend on the size of the emboli. Patients may have no symptoms or may have complete arrest due to right-sided heart failure.
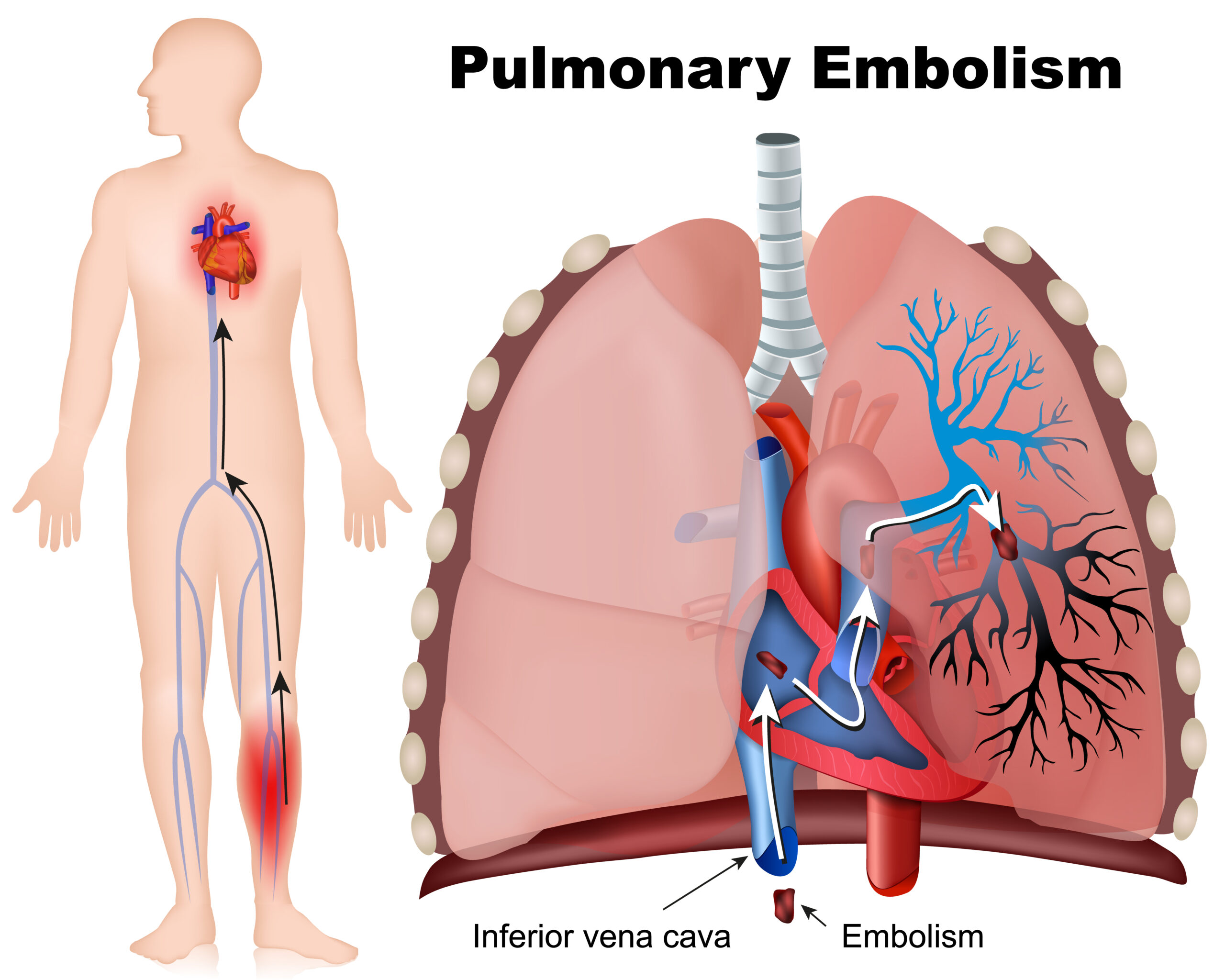
Pulmonary embolism is a blood clot that travels to the lungs.
Additionally, the deficit in blood flow to the lungs can lead to downs stream effects such as hypoxemia and pain as well as upstream effects such as heart failure. PE is life-threatening; up to ¼ of symptomatic patients will have sudden death, and while major PE with large thrombi, which occurs in 5% of patients, will lead to death in 50% of the patients. Overall, PE is associated with a 15% mortality at 3 months.
PE presentation can vary due to the degree. The classic presentation is dyspnea, hemoptysis, and pleuritic chest pain; however, this is only present in 20% of patients. This presentation is actually typical of less severe PE that causes infarcts in the lung and inflammation of the pleura. Patients may have an associated effusion of chest x-ray. Commonly, there will be tachypnea (96%) or dyspnea (82%), and chest pain is found in about half. About 90% will have symptoms but will be stable hemodynamically.
Related Video: Hs and Ts – Pulmonary Embolism
ECG changes
ECG findings for PE are variable. Typically, there will be tachycardia as well as nonspecific ST changes. Some patients may have atrial fibrillation or RBBB due to right heart failure.
Cardiac Biomarker Changes
Both troponins, and less commonly, CK-MB can be elevated in PE. This is likely due to severe PE with associated right heart failure. The combination of obstruction of the right ventricle and the large PE can lead to right-sided ischemia and infarct that raises cardiac biomarkers. As a result, patients in this group can benefit from fibrinolytic therapy with tissue plasminogen activator (tPA). The research is controversial, however, as treatment is associated with up to 3% risk of intracranial bleeds and long-term outcomes are unclear.
ACLS for PE
In patients with cardiac arrest known to be due to PE, treatment should be directed to resolving the PE via thrombolysis or surgical/mechanical embolectomy. There is no evidence that one of these options is better than the rest, rather treatment depends on resource availability and patient characteristic (such as contraindications to thrombolysis). Note that since severe PE is associated with high mortality, normal contraindications to thrombolysis may be overridden to improve survival.
In patients with cardiac arrest suspected to be due to PE, thrombolysis is an option. The research is not specific as to inclusion criteria, not the timing, dosing, or specific therapy. There is no good data showing benefit for surgical/mechanical embolectomy in cases of suspected PE.
Key Takeaway
In patients with normal angiography but elevated troponin, consider PE as a possible etiology.
How does ACLS improve PE Outcomes?
An echocardiogram may be useful for managing acute PE. About 5% of PE patients will be able to receive fibrinolytics or embolectomy due to associated shock. Additionally, 40% will have dysfunction of the right ventricle. An echocardiogram can be used to identify ventricular dysfunction via poor wall motion and maintained arterial pressures. While the best treatment of these patients is not known, there is evidence that fibrinolysis in associated dysfunction of the right ventricle may reduce repeated episodes of PE. Identifying these findings on echocardiogram can be used to help guide treatment or at least ensure heightened monitoring of coagulation and alternate treatment options.
Pericarditis and Cardiac Tamponade
In cardiac tamponade, there is excess fluid surrounding the heart in the pericardium. It is commonly associated with pericarditis, but can also occur in cancers, uremia from renal failure, aortic dissections, and endemic tuberculosis. This condition can easily be mistaken for ACS due to similar presentations and ECG findings. Pericarditis is associated with inflammation of the cardiac tissue and therefore increased bleeding risk. Erroneous treatment with heparin or fibrinolytics can worsen the disease process and lead to bleeding.
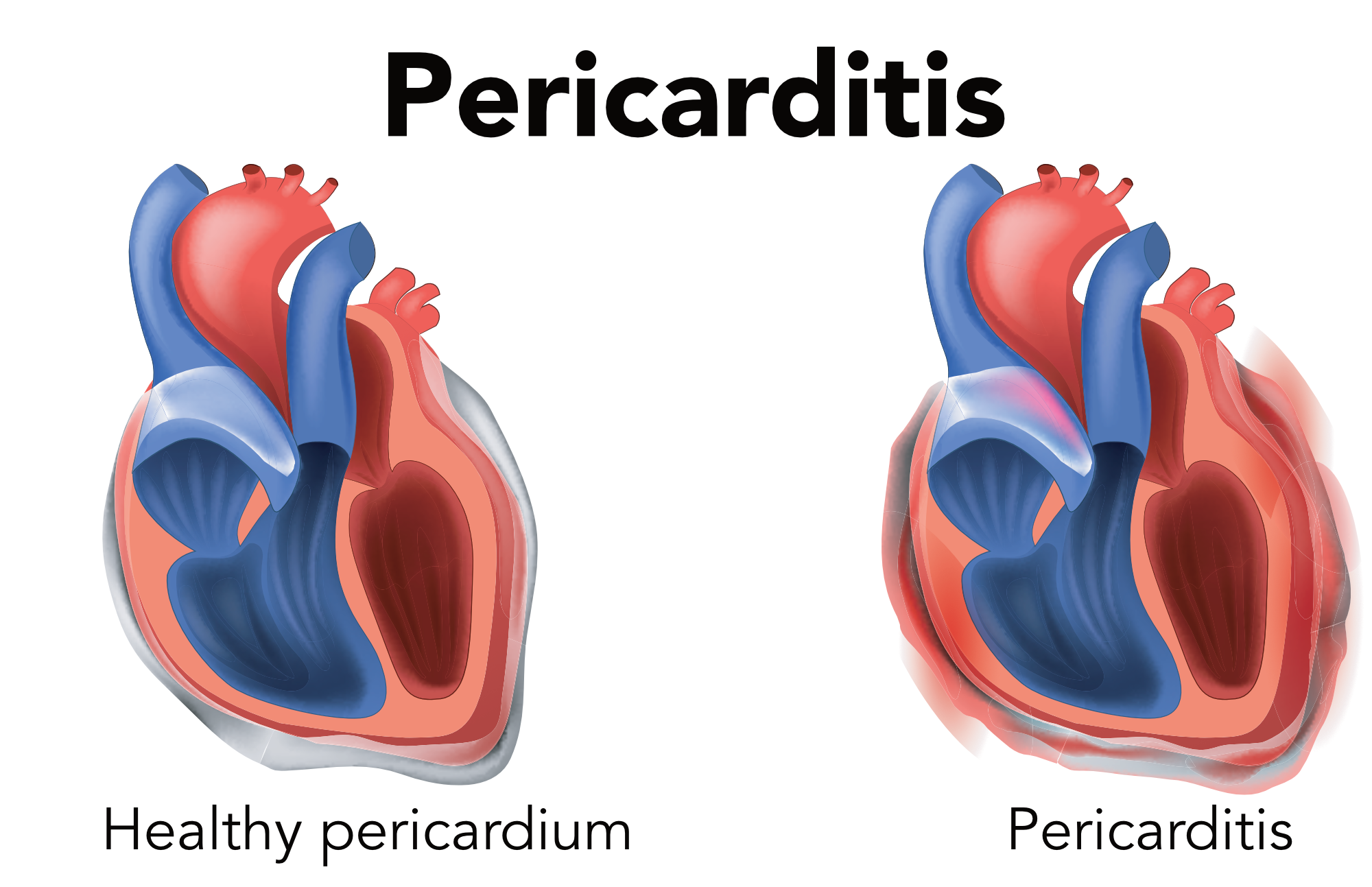
Pericarditis is pericardium inflammation.
Since the pericardium and myocardium are close, pericarditis is often associated with myocardial inflammation. As a result, patients can have elevated cardiac biomarkers. Typically, in the normal heart, there are only about 20 ml of serous fluid in the pericardial space. Up to 120 ml of fluid may be asymptomatic. With more fluid, pressure around the heart increases rapidly, which can lead to significant outflow obstruction of the heart and reduced contractions. Patients will have a drop in cardiac output as well as blood pressure. The rate of fluid accumulation is more important than the amount. Rapid accumulation, such as in the case of trauma or left ventricular cardiac rupture, can lead to catastrophic outcomes and acute heart failure. Outcomes may be better if there is perforation of the right side of the heart, i.e., after placing pacers, catheterization of the pulmonary artery, or other interventions of the right heart. This is due to the lower right-sided pressures that can be temporized until treatment with pericardiocentesis. Health care providers must be aware that cardiac interventions, including PCI and angiography, can lead to coronary artery dissection or perforation and can cause delayed cardiac tamponade.
The most common symptom of pericarditis is chest pain. Symptoms can be quite similar to ACS with central chest pain that radiates to the arm, neck, or back and that is stabbing or sharp. Additionally, there may be increased pain with inspiring or movement, and that is improved with sitting forward. Associated symptoms include cough, low-grade fever, and pain with swallowing.
As the disease progresses, patients may note worsening dyspnea, anxiety, fatigue, and signs of lowered perfusion. Patients with a rapid increase in pericardial fluid or a constricted pericardium will have more symptoms. The three classic symptoms of tamponade are hypotension, distension of jugular veins, and muffled heart sounds. It can be differentiated from pulmonary conditions due to the usually normal lung sounds. Pulsus paradoxus, in which systolic BP decreases with inspiration, is common. Typically, the drop in systolic BP will be 8-10 mm Hg or more. Note that pulsus paradoxus also occurs in pulmonary conditions.
Related Video: Hs and Ts – Cardiac Tamponade
ECG changes
ECG changes in ACS and pericarditis can be quite similar. However, ST elevation in pericarditis is typically in many leads, while it is typically grouped in regional leads in ACS. Pericarditis also usually has a shortened PR interval. Alternatively, there can be nonspecific ECG findings. An echocardiogram is particularly useful for diagnosing cardiac tamponade.
Typically, there is a progression of ECG findings into four progressive stages:
- 1st stage: Upward and concave ST elevation in cardiac leads except for V1. This is in the first hours of disease onset and can last for days.
- 2nd stage: flattening of the T wave and normalization of ST-elevation
- 3rd stage: inversion of T waves. (no associated Q waves)
- 4th stage: normalization of EKG
Note that the ECG complex (P wave, QRS, T wave) will vary over time. This is due to the movement of the heart towards and away from the leads as it moves in the surrounding fluid.
Tension Pneumothorax
This condition is caused by lung or bronchial injury that causes air leak. As this trapped air increases in the pleura of the lungs, pain and pressure increase. The lung, as well as the heart, is pushed away from the pneumothorax with increasing pressure. Lung compression further worsens hypoxia and can ultimately lead to lung failure. As the heart and vena cava are compressed, preload is decreased as is cardiac output. Patients may develop significant hypoxia, hypotension, and shock leading to death.
Pneumothoraxes can develop spontaneously, which is considered primary disease. This usually does not lead to clinical tension pneumothorax. Secondary causes include interventional procedures in the chest, neck or abdomen as well as central catheters, aggressive ventilatory assistance (especially if high peak inspiratory pressures or underlying lung disease), or significant chest trauma.
The classic finding is dyspnea that can lead to respiratory failure. Other common signs include poor chest movement, increased tympany of the ipsilateral chest wall, decreased lung sounds, and hypoxemia.
While CXR confirms the diagnosis, the real diagnosis should be made clinically as a delay for a CXR can be fatal for the patient. Signs that are particularly problematic for arrest include
- Absence of ipsilateral lung sounds
- Hypotension
- Cyanosis
- Respiratory distress/arrest
- Distension of jugular veins
- Pulsus paradoxus
- Deviation of the trachea
Related Video: Hs and Ts – Tension Pneumothorax
ECG Changes
The findings can be variable. Many patients will have tachycardia. There may be deviation of the electrical axis which will depend on the side of the pneumothorax (i.e., clockwise or counterclockwise)
Tension Pneumothorax in Left Lung